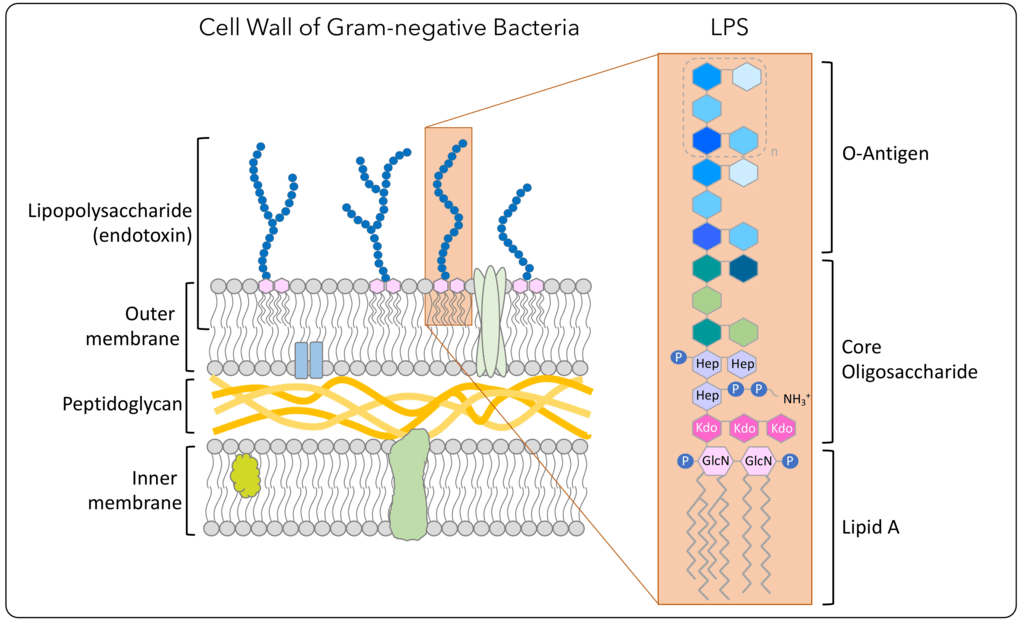
Bacterial Endotoxin Test History . Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Biomedical instrumentation & technology ,.
from onelab.andrewalliance.com
The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. Biomedical instrumentation & technology ,. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal).
EndoLISA® Detection Assay Protocol OneLab
Bacterial Endotoxin Test History The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Biomedical instrumentation & technology ,. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72:
From www.virtuosivr.com
Bacterial Endotoxin Testing Virtuosi Bacterial Endotoxin Test History Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. Biomedical instrumentation & technology ,. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in. Bacterial Endotoxin Test History.
From www.nelsonlabs.com
Bacterial Endotoxins Test (BET) Services Nelson Labs Bacterial Endotoxin Test History The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: Biomedical instrumentation & technology ,. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside. Bacterial Endotoxin Test History.
From www.bjaed.org
Bacterial endotoxins and exotoxins in intensive care medicine BJA Bacterial Endotoxin Test History Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Since 2004 a bacterial endotoxin test based. Bacterial Endotoxin Test History.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test History Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Biomedical. Bacterial Endotoxin Test History.
From www.researchgate.net
(PDF) Comparison of bacterial endotoxin testing methods in purified Bacterial Endotoxin Test History A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Biomedical instrumentation & technology ,. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Testing for endotoxins (a toxin. Bacterial Endotoxin Test History.
From bioindus.com.pk
Bacterial Endotoxin Test (Chromogenic Method) Bioindus Bacterial Endotoxin Test History Biomedical instrumentation & technology ,. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Testing for endotoxins (a toxin present. Bacterial Endotoxin Test History.
From ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Testing Bacterial Endotoxin Test History A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: Biomedical instrumentation & technology ,.. Bacterial Endotoxin Test History.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test History The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Biomedical instrumentation & technology ,. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has. Bacterial Endotoxin Test History.
From eagleanalytical.com
Bacterial Endotoxin Test USP 85 Eagle Analytical Bacterial Endotoxin Test History Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been. Bacterial Endotoxin Test History.
From www.scribd.com
Bacterial Endotoxin Test 14 03 17 PDF PDF Sensitivity And Bacterial Endotoxin Test History A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: Biomedical instrumentation & technology ,. Since 2004 a. Bacterial Endotoxin Test History.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test History A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Biomedical instrumentation & technology ,. The association. Bacterial Endotoxin Test History.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test History The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Biomedical instrumentation & technology ,. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Since 2004 a bacterial. Bacterial Endotoxin Test History.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test History The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Biomedical instrumentation & technology ,. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with. Bacterial Endotoxin Test History.
From www.biomerieux-industry.com
BACTERIAL ENDOTOXIN TESTING 10 reasons to choose factor C Bacterial Endotoxin Test History Biomedical instrumentation & technology ,. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. The federal register, january 18, 1980, proposed guidelines for determining endotoxins with. Bacterial Endotoxin Test History.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test History The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The limulus/tachypleus amebocyte lysate (lal or tal) method for the detection of bacterial endotoxin was developed in the 1970s,. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an.. Bacterial Endotoxin Test History.
From www.scribd.com
Bacterial Endotoxin Testing in the Laboratory Lipopolysaccharide Bacterial Endotoxin Test History The federal register, january 18, 1980, proposed guidelines for determining endotoxins with the limulus amebocyte lysate test (lal). Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. Biomedical instrumentation & technology ,. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein. Bacterial Endotoxin Test History.
From www.europeanpharmaceuticalreview.com
ISO publishes standard on testing bacterial endotoxins Bacterial Endotoxin Test History Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. Since 2004 a bacterial endotoxin test based on recombinant factor c (rfc), the endotoxin sensor protein inside of lal, has been used as an. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami. Bacterial Endotoxin Test History.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test History Testing for endotoxins (a toxin present in a bacteria cell that is released when the cell disintegrates) in injectable biologics was. A report on the methods, background, data, and regulatory history of extraction recovery efficiency. The association for the advancement of medical instrumentation (aami) recently published a standard designated ansi/aami st 72: The limulus/tachypleus amebocyte lysate (lal or tal) method. Bacterial Endotoxin Test History.